Background Chimeric Antigen Receptor T-Cell therapy (CAR-T) is increasingly used for older adults with hematologic malignancies. Real-world outcomes suggest chronological age alone does not have a strong impact on CAR-T outcomes or associated adverse effects, including cytokine release syndrome (CRS) and ICANS. A subset of CAR-T-eligible older adults have comorbidities managed with deliriogenic medications including opioids, benzodiazepines, and sedatives. Their effects may be exacerbated during prolonged hospitalizations for CAR-T therapy, precipitating delirium, which has clinical overlap with ICANS including inattention, altered sensorium, and reduced responsiveness. We hypothesized that exposure to high-risk deliriogenic medications may have an impact on observed ICANS incidence in older adults undergoing CAR-T therapy.
Methods We performed a single-center retrospective analysis of all patients age 60 or older who received a FDA-approved CAR-T product for a hematologic malignancy at two UCLA Health hospitals from 8/2018 to 12/2022. We collected exposure to deliriogenic medications including opioids, benzodiazepines, sedatives/hypnotics, and anticholinergics prior to and during CAR-T hospitalization. Correlation with incidence of CRS and ICANS of any grade was modeled using logistic regression. If exposure to high-risk medication was found to be statistically significant, correlation with CRS and ICANS by each individual class of high-risk medications was analyzed. A multivariable logistic regression was performed including each predictor of CRS or ICANS that was statistically significant at the p<0.1 level in univariable regression.
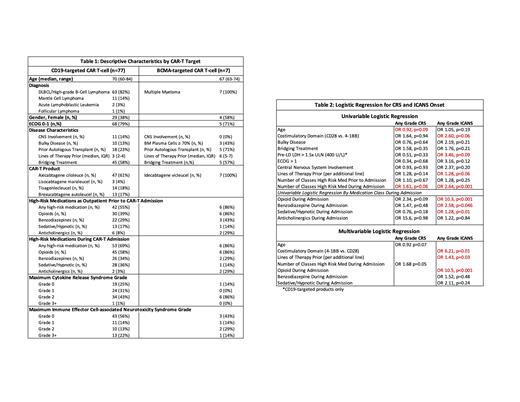
Results A total of 84 patients age 60 or older received an FDA-approved CAR-T product for a hematologic malignancy at our institution between 8/2018 to 12/2022. Patients ranged in age from 60 to 84 years (median 70). Baseline characteristics for all patients by cellular therapy target are shown inTable 1. The most common histology included was DLBCL (75%), followed by MCL (13%), MM (8%), ALL (2%), and FL (1%). With a median follow up of 9.6 months, CRS and ICANS of any grade were experienced by 76% and 45% of total patients with a median time to onset of 4 days and 5 days, and grade 3 or higher CRS and ICANS was experienced by 1% and 17% of patients, respectively. Most patients had exposure to a high-risk deliriogenic medication prior to and during their hospitalization for CAR-T infusions (57% and 74%, respectively).
Age, performance status, and deliriogenic medication use prior to admission were not correlated with incidence of CRS or ICANS as shown in Table 2. However, exposure to high-risk medications during hospitalization was highly correlated with ICANS incidence (OR 2.64, p<0.001). In a multivariable model CAR-T product costimulatory domain (4-1BB vs. CD28, OR 6.21, p= 0.01), number of prior lines of therapy (OR 6.21, p=0.03), and exposure to opioids during admission (OR 10.5, p<0.001) were correlated with incidence of ICANS.
Conclusion In our single-center real-world analysis of older adults receiving a FDA-approved CAR-T therapy, the CAR-T product infused, number of prior lines of therapy, and exposure to opioids during CAR-T hospitalization were correlated with incidence of ICANS. Increased incidence and severity of ICANS depending on CAR-T product has been well-described, but interestingly, our data suggest use of opioids in the immediate post-CAR-T infusion setting may also be a significant risk factor for ICANS. Limitations to our analysis include single-center practice patterns, unclear timing of exposure to deliriogenic medications to onset of ICANS, burden of comorbidities, and lack of baseline standardized cognitive and geriatric assessments in our elderly cohort. In addition to careful selection of CAR-T product in older adults, our findings support minimization of high-risk deliriogenic medications in the immediate post-CAR-T inpatient setting to mitigate risk of ICANS in this vulnerable patient population. Effects of ICANS and its treatments on outcomes and survival will be reported as this cohort matures.
Disclosures
Timmerman:Oncovalent Therapeutics: Consultancy; BMS: Other: Travel/Accommodations/Expenses, Research Funding; Merck & Co., Inc.: Research Funding; DAVA Oncology: Consultancy; Kite/Gilead: Consultancy, Honoraria, Research Funding. Oliai:Arog: Research Funding; Jazz Pharmaceuticals: Research Funding; Orca Bio: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Seagen: Research Funding. de Vos:BeiGene: Consultancy. Larson:AbbVie: Research Funding; 1200 Pharma: Current equity holder in private company; TORL Biotherapeutics: Current equity holder in private company; Bioline: Research Funding; Allogene: Research Funding; Ionis: Research Funding; Pfizer: Research Funding; ImmPACT Bio: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Bristol Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal